How Many Electrons Does Oxygen Have?
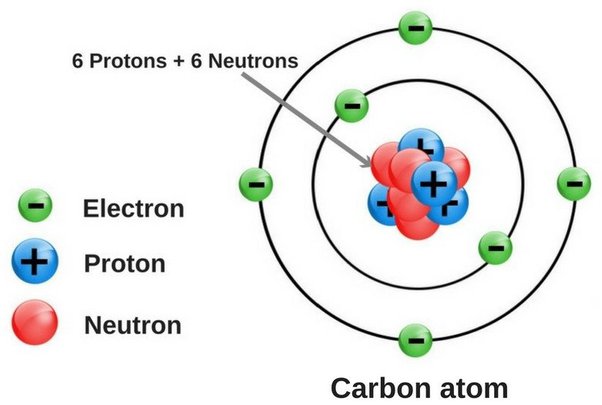
Table of Contents
Which Is The Most abundant And Simplest Element
How many electrons does oxygen have? The answer may surprise you. There are actually seven in all. That’s quite a lot. However, you might still be curious about the amount of valence electrons in oxygen. So, let’s begin with the basic definition. The simplest form of oxygen is a gas, with one atom per molecule. This makes it the simplest element. It also happens to be the most abundant.
Number Of Electron In Oxygen
Oxygen has 8 electrons in total, with six in its second shell and two in the 4th. The rest of the atom is made up of nuclear electrons. In the periodic table, each atom has a number of valence and core valence atomic electrons. The atomic number of an atom is its valence electrons, while its p and d shells have the same number of valence and core electrons.
How Many Valence And Core Electrons Does Oxygen Have
In addition to having six valence electrons, oxygen also has two core electrons. This means that the atom has two valence electrons and two core electrons. These electrons are not reactive and are not involved in chemical reactions, but they are not present in the oxygen atom. That’s why it’s important to know how many electrons oxygen has in order to properly analyze a substance.
Outer And Inner Shells Of Oxygen
When it comes to valence electrons, oxygen has two. This means that it has six outermost shell electrons. It’s important to note that the innermost shell of oxygen has two core or “core” valence electrons. The reason for this is because the oxygen has a large outer shell and would like to have eight electrons in it. Ultimately, this would create a second-shell atom with two outermost shells.
Number Of Neutron And Proton In Oxygen
As you can see, oxygen has eight electrons. In fact, the oxygen atom has six valence and two core electrons. The number of valence and core electrons in an atom is equal to the number of principal groups in the atom. Moreover, each element has a different number of neutrons and protons. This means that an oxygen molecule has eight valence and two core-shells.
What are Valence Electrons
The valence electrons are the ones that surround the oxygen nucleus. The oxygen atom has six valence electrons. The other two are known as the core-shell. The outer shell is filled with four valence and three core-shells. In general, the outer shell of an atom has six valence and octet. In contrast, the core-shells of an atom contain only two core electrons.
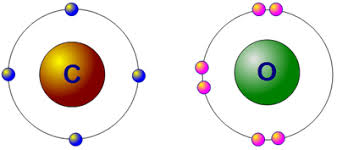
Octet Electrons In Oxygen
A good example of this is the number of valence electrons in an atom. The number of valence electrons is the outermost electron of the oxygen atom. This is the outermost electron of oxygen. The core-shell electrons are the ones that aren’t involved in reactions. Usually, an atom with a full outer shell will have eight valence or octet electrons.
Neutral Oxygen Atom Has How Many Valence Electrons
A neutral oxygen atom has eight valence electrons. The other six electrons are core-shell. The outermost shell has six valence and four core-shells. The oxygen atom has six valence-shells and two core-shells. The octets of a hydrogen atom are surrounded by a hydrogen atom. In the Lewis structure, an atom is filled with a certain number of electrons.
Oxygen is a non-metallic element
Oxygen is a non-metallic element and is part of the group-16 family. Its valence-shell electrons determine the properties of an atom. A hydrogen atom has two and an oxygen atom has eight. Its valence electrons are responsible for its ability to bond with other elements. While the atomic number of an element can be very important, a water molecule will have only two or three of them in its outermost shell.






